So, you want to know why lithium-ion batteries explode? It’s a pretty scary thought, right? These little powerhouses are in everything from our phones to our electric cars, and the idea of them spontaneously combusting is definitely unsettling. Let’s dive into the reasons behind this potentially dangerous phenomenon.
The causes of lithium-ion battery explosions are multifaceted, and it’s not always a single, easily identifiable culprit. Think of it like a complex chain reaction, where one thing leads to another, ultimately resulting in a fiery outcome. One major factor is internal short circuits. These can happen due to manufacturing defects, where tiny metal particles might bridge the gap between the positive and negative electrodes inside the battery. Imagine a tiny spark igniting a highly flammable material – that’s essentially what happens. These short circuits can generate significant heat, and that heat is the key player here.
Another significant cause is overcharging. When you push a lithium-ion battery beyond its designed capacity, you’re essentially forcing it to work harder than it should. This extra stress leads to increased internal pressure and heat buildup. Think of it like inflating a balloon past its limit – eventually, it’ll burst. Similarly, an overcharged battery can overheat, leading to thermal runaway. This is a chain reaction where the heat generated by the initial overcharging triggers further chemical reactions, releasing even more heat, and escalating the process until it culminates in an explosion or fire. This is why using reputable chargers and avoiding leaving your devices plugged in overnight is so crucial.
External factors also play a role. Overheating from external sources, like leaving your phone in direct sunlight for an extended period or subjecting a battery pack to extreme temperatures, can also trigger thermal runaway. Physical damage, such as punctures or crushing, can also compromise the battery’s integrity, leading to internal short circuits and subsequent explosions. Think about the delicate internal structure of a lithium-ion battery – even a small puncture can disrupt the carefully balanced chemical environment inside.
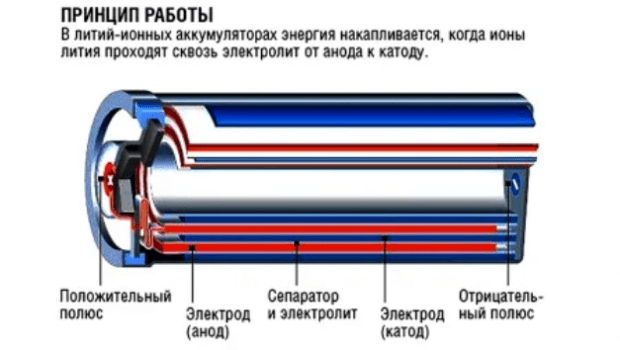
Now, how does a rechargeable battery spontaneously combust? It’s not as spontaneous as it sounds. It’s a process, a chain of events. As we’ve discussed, internal short circuits, overcharging, and external factors all contribute to the buildup of heat. This heat triggers a process called thermal runaway, a self-accelerating chain reaction where the heat generated by the initial problem causes further chemical reactions, releasing even more heat. This escalating heat eventually leads to the ignition of the flammable electrolytes within the battery, resulting in a fire or explosion. The electrolytes are the liquid or gel that carries the ions between the electrodes, and they’re highly flammable. So, the combination of intense heat and flammable electrolytes creates a perfect storm for a catastrophic event. The speed at which this happens can vary, but the underlying principle remains the same: excessive heat and the flammable nature of the battery’s components. Understanding these factors is key to preventing these incidents.
So, we’ve all heard the horror stories – phones bursting into flames, laptops spontaneously combusting. It’s terrifying, and it all comes down to those ubiquitous lithium-ion batteries. But why do they explode? It’s not quite as simple as «they’re just dangerous.» There are a lot of factors at play.
Let’s talk about some other causes of lithium-ion battery explosions besides just inherent instability. Overcharging is a HUGE one. Pushing more energy into a battery than it’s designed to handle creates internal pressure, generating heat. This heat can trigger a chain reaction, leading to thermal runaway – a process where the heat generated accelerates the chemical reactions within the battery, leading to a rapid increase in temperature and pressure, ultimately resulting in an explosion or fire. Think of it like a pressure cooker that’s been left on the stove for too long. Similarly, short circuits can cause a massive surge of current, generating intense heat and leading to the same catastrophic outcome. Damage to the battery itself, like punctures or crushing, can also compromise its internal structure, leading to short circuits and thermal runaway. Even extreme temperatures, both hot and cold, can stress the battery and make it more prone to failure. Exposure to moisture can also cause internal shorts and degradation, increasing the risk of explosion. It’s a complex interplay of factors, not just one single culprit.
Now, what are manufacturers doing to prevent these disasters? Well, a lot, actually. They’re using sophisticated safety mechanisms built right into the batteries themselves. These include things like protective circuits that monitor voltage, current, and temperature. If any of these parameters exceed safe limits, the circuit will cut off the power supply, preventing further damage. They also incorporate things like pressure relief valves, which are designed to release excess pressure before it builds up to dangerous levels. These valves are crucial in preventing explosions, allowing for a controlled release of gases instead of a violent rupture. Furthermore, manufacturers are constantly improving the battery chemistry itself, making them more stable and less prone to thermal runaway. They’re also focusing on better battery management systems (BMS) which are essentially sophisticated computer chips that constantly monitor the battery’s health and performance, adjusting charging and discharging parameters to optimize safety and longevity. It’s a constant evolution, a race to make these powerful energy sources safer.
But what can you do to avoid a battery pack from spontaneously combusting? First and foremost, only use chargers and accessories specifically designed for your device. Using incompatible chargers can lead to overcharging and damage. Avoid leaving your devices plugged in overnight or for extended periods after they’re fully charged. This reduces the risk of overcharging. Keep your batteries away from extreme temperatures – don’t leave them in direct sunlight or in freezing conditions. Handle your batteries with care; avoid dropping, puncturing, or crushing them. And finally, pay attention to any signs of damage or unusual behavior. If your battery is swelling, overheating, or leaking, stop using it immediately and replace it. Your safety is paramount. It’s about responsible use and awareness.
And finally, let’s touch on some related news. We’ve seen numerous recalls of electronic devices due to battery-related issues. These recalls highlight the ongoing challenges in ensuring battery safety. Staying informed about these recalls and following manufacturers’ safety guidelines is crucial. The news often highlights the severity of these issues, reminding us of the importance of responsible battery handling and the ongoing efforts to improve battery safety technology. It’s a constantly evolving landscape, and staying informed is key.